Theme 2 Virulence of opportunistic bacteria belonging to the Burkholderia cepacia complex
oVERVIEW
Infections of bacteria belonging to the Burkholderia cepacia complex
Bacteria belonging to the Burkholderia cepacia complex (Bcc), including one of the more virulent Burkholderia cenocepacia species, are are a major cause of mortality and morbidity in cystic fibrosis patients. Infection can be asymptomatic but often results in chronic progressive worsening of lung function and sometimes acute fatal necrotizing pneumonia and sepsis, termed Cepacia Syndrome. The inherent resistance of the bacterium to clinically used antibiotics makes it is impossible to control the infection. Opportunistic infections with Bcc bacteria are emerging also in non-CF conditions. The molecular mechanisms that allow B. cenocepacia to cause disease in humans are still largely unknown. B. cenocepacia is an intracellular pathogen.
Zebrafish as an infection model. We are using the zebrafish larvae to better understand the role of intramacrophage stages in virulence during acute and persistent infections in vivo, and the transition between chronic and acute disease. Our studies focus on host autophagy and pro-inflammatory signaling, and we are using high throughput techniques to identify bacterial and host factors that are critical for the intracellular survival and replication of the bacteria.
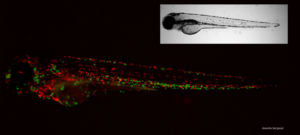
We have established the zebrafish (Danio rerio) embryo model to study Bcc virulence. The zebrafish has become a potent biomedical research model that has revolutionized our knowledge of the interaction of microbes with host phagocytes and the immune system through powerful live imaging and genetic tools. The transparent fish embryos allow easy real time analysis of infection with fluorescent bacteria, and in combination with transgenic reporter fish this allows for remarkable imaging of the behavior of host cells during infection. Most importantly, an innate immune system, with a high degree of similarity to that of mammals is already developing in the young embryo.
In zebrafish embryos, the epidemic CF isolate B. cenocepacia K56-2 induces an acute pro-inflammatory infection that becomes rapidly fatal. Intravenously injected bacteria are mainly taken up by macrophages in which they are able to survive and efficiently replicate (Vergunst et al 2010), prior to dissemination and induction of systemic fatal infection. In contrast, other isolates, notably B. stabilis LMG14294 and B. vietnamiensis FC441, cause persistent infection, characterized by low but steady bacterial numbers in macrophages. We have recently found that macrophages are critical for bacterial replication and development of fatal pro-inflammatory responses in zebrafish embryos, in agreement with clinical observations. The model is particularly amenable to study in more detail these intracellular stages in vivo, and the effect of bacterial gene expression on host immune response.
Research Projects
We are using the zebrafish embryo as an animal model to study the capacity of intracellular Bcc to arrest maturation of (auto)phagosomes and create an intracellular replication niche, and to study and identify host factors involved in persistent and acute phases of the disease.
- Analysis of intracellular bacterial stages and identification of bacterial and host factors involved in infection.
- Burkholderia Adaptation to multiple hosts:exploring frontiers between eukaryotic mutualists and pathogens. ANR project
- Analysis of the role of the lysR transcriptional regulator ShvR in acute infection
Grants
ANR BURKADAPT, Coordinator IRD (Montpellier) (2019-2023)
Marie-Curie International training network (2012-2015) ![]()
Vaincre la Mucoviscidose (2016-2018), link
Fondation pour la recherche médicale (2010, 2016) link
Région de Languedoc-Roussillon (2010-2012, grant Chercheuse d’avenir, Annette Vergunst)